This module describes the skills, knowledge, and attitude required to Manage a watershed. At the
end of this module the learner will be able to characterize watershed, develop watershed
management plan, build a watershed management partnership, implement a watershed
management plan, and evaluate watershed management activities.
- Teacher: Xavier URAYENEZA

This module describes the skills, knowledge, and attitude required to manage a watershed. At the end of this module the learner will be able to:
Characterize the watershed,
Develop watershed management plan,
Build a watershed management partnership,
Implement a watershed management plan, and
Evaluate watershed management activities.
- Teacher: MIZERO JULES
This module describes the skills, knowledge, and attitude required to apply Hydro-Geology. It is intended for learners pursuing a Bachelor of Technology in Irrigation and Drainage Technology. At the end of this module, the learner will be able to characterize the geological formations of the site, monitor groundwater quality and quantity, and exploit groundwater resources.
- Teacher: Dieudonne Uwizeyimana

This module is designed to provide undergraduate (B-Tech) students with the essential knowledge and skills necessary for conducting effective research. It comprehensively addresses the entire research process, from problem formulation through data collection, analysis, and writing an academic report, laying a solid foundation for their independent research projects. By the end of this module, students will be able to navigate the complexities of research and apply their learning to real-world research endeavors.
The number of credits of this module is 10 with 100 learning hours. The module is organized into three (3) elements of competence: Identify research gaps, Develop a research project, Write an academic research report. The topics are organized in a sequential manner in terms of the level of complexity of the subject matter, and the author has ensured that the previous topic introduces and complements the succeeding topic, thus making the subject matter of the latter easier to follow and comprehend.
- Teacher: MIZERO JULES
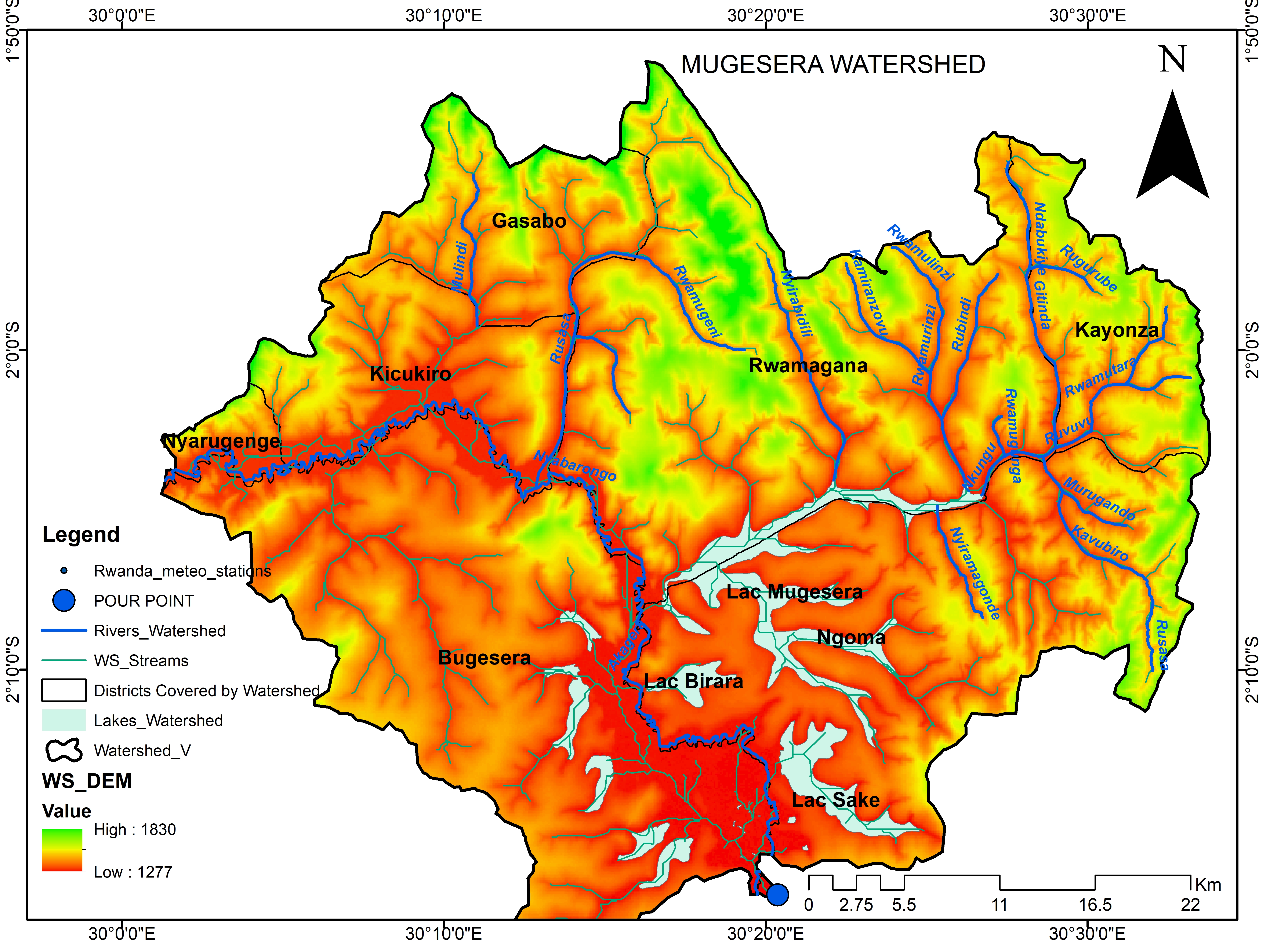
This module equips you with the skills, knowledge, and mindset to apply Geographical Information Systems (GIS) and hydrological models in irrigation and drainage. You will learn how to collect and analyze spatial data, model hydrological processes, and validate results to meet technical requirements. By the end, you will be able to confidently use GIS and modeling tools to support effective water resource management.
- Teacher: Jean Nepomuscene Nsengiyumva


